Finally put the lone pairs on the o. For the CH3OCH3 Lewis structure we have a total of 20 valence electrons.
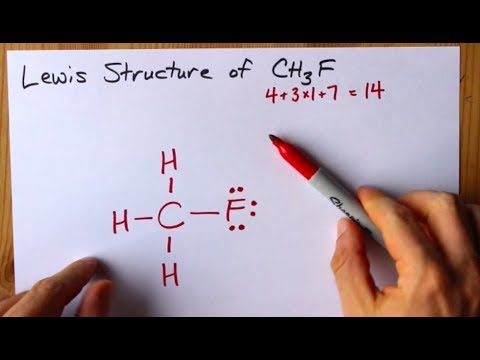
How To Draw The Lewis Structure Of Ch3f Fluoromethane Youtube
Lewis diagrams are used to show the connectivity between atoms in a molecule they tell you nothing about the arrangement of the atoms of a molecule within.

. This is the CH3OCH3 Lewis structure. Hydrogen always goes on the outside of Lewis structures. Representing two pairs of electrons.
Similarly replace the double bond with. CH3F lewiss structure contains one carbon attached with three hydrogen atoms and one fluorine atom. Include all hydrogen atoms and nonbonding electrons.
When we are drawing a CH3OCH3 molecule we must remember that it consists of the ether R-O-R here RR group. General Concepts 9. For CH3F we have a total of 14 valence electrons.
Methyl fluoride is the member with the lowest mass in the HFC series. How do you determine the Lewis structure of CH3OCH3. 1 Chemical Foundations 2 Atoms Molecules And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding.
Show the formal charges of all atoms. CH3OH Molecular Geometry. The molecular geometry of the molecule is tetrahedral that is sp3 hybridization.
Well put a Carbon then Oxygen then another Carbon and then our Hydrogens theyll go around the outside like so. By Staff Writer Last Updated March 31 2020 The Lewis structure of CH3OCH3 is dimethyl ether the simplest ether in existence. The way this is written really tells us how were going to draw the Lewis structure.
KOH Draw the Lewis dot structure for KOH. Problem 84 Hard Difficulty. In the Lewis structure the central oxygen atom is bonded to two carbon atoms which have little or no electronegativity.
The structure of the organic compound contains a total of 20 valence electrons. Being the least electronegative carbon is the central atom in CH3F lewiss structure. Answer to Draw a Lewis diagram for CH3C2H Draw a Lewis structure for C3H6 Question Draw a Lewis diagram for CH3C2H Draw a Lewis struct.
What is the Lewis dot structure of bicarbonate ion. As the Carbon has four valence electrons that. There is a total of 4 bonded pairs and 3 lone pairs present in the CH3F lewis dot structure.
Best Writers chemistry Question Draw a Lewis diagram. We can see that oxygen has acted as the central atom in. This is the Lewis structure for CH3F.
CH3F is a liquefiable flammable gas with a molecular weight of 37015 gmol. A step-by-step explanation of how to draw the CH3CH3 Lewis Dot StructureFor the CH3CH3 structure use the periodic table to find the total number of valence. The gas has a pleasant odor and at high concentrations the smell is similar to.
See the Big List of Lewis Structures. So the skeletal formula for dimethyl ether looks like this. To draw the lewis dot structure of CH3F follows some simple steps.
You can start by drawing the structural formula explicitly rendering the hydrogen atoms. Carbons valency is 4 4 single bonds2double bonds Hydrogens is 1 1 single bond Oxygens is 2 2 single bonds1 double bond So for CH3OCH3 each Carbon atom bonds to three Hydrogen atoms and the central Oxygen atom. However the carbonate anion CO32- does have a Lewis dot structure.
Answer 1 of 2. For the CH3OCH3 structure use the periodic table to find the total number of valence electrons for the CH3OCH3 molecule. Then replace the single bonds with.
While drawing the Lewis structure for CH3OH you will notice that the Carbon atom will have three bonds with three hydrogen atoms and one bond with the Hydroxyl Group. Once we know how many valence electr. Now that we know the Lewis structure of CH3OH it is easy to depict the compounds molecular geometry.
For the molecule CH3OCH3 dimethyl ethera Draw the Lewis structure of the moleculeb Identify where the lone pair electrons are locatedc Identify the n. It is an ionic compound so it would not have a Lewis dot structure. First determine the valency of each atom involved.
Draw the Lewis structure for each organic compound from its condensed structural formula. To change the symbol of an atom double-click on the atom and enter the letter of the new atom. Methyl fluoride is a colorless gas.
Representing a pair of electrons. And then Carbon is less electronegative than Fluorine so lets put the Carbon in the center and the Hydrogens on the outside there and the Fluorine on the top.
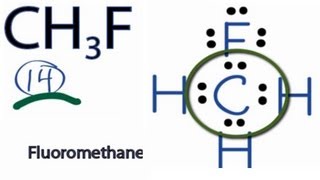
Ch3f Lewis Structure How To Draw The Lewis Structure For Ch3f Fluormethane Youtube

How To Draw Ch3f Lewis Structure Science Education And Tutorials

Ch3f Lewis Structure Molecular Geometry Hybridization Mo Diagram Tuktuk Study

Ch3f Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

How To Draw Ch3f Lewis Structure Science Education And Tutorials

Ch3f Lewis Structure Molecular Geometry Bond Angle Polarity Electrons

Ch3f Lewis Structure How To Draw The Lewis Structure For Ch3f Fluormethane Youtube

Ch3f Lewis Structure Molecular Geometry Bond Angle Polarity Electrons
0 comments
Post a Comment